Assuming constant pressure, ΔH is a measure of the change of heat energy during a reaction. Since ΔH is negative, energy was released during the reaction therefore this reaction is exothermic. Suppose 20 g of H2O2 react. Will heat be released or absorbed?
QUIZ RATE OF REACTION Quiz Grade 12 Chemistry Quiz WITH ANSWERS Ver. #8 | Teaching Resources
This reaction released 48.7 kJ of heat when 3.12 g of glucose was burned. Exercise 5.3.5 5.3. 5. When 0.963 g of benzene, C 6 H 6, is burned in a bomb calorimeter, the temperature of the calorimeter increases by 8.39 °C. The bomb has a heat capacity of 784 J/°C and is submerged in 925 mL of water.

Source Image: snexplores.org
Download Image
A chemist measures the energy change ∆H during the following reaction \ce 2Fe2O3 (s)\rightarrow 4FeO (s)+O2 (g) 2FeX 2OX 3(s) → 4FeO(s)X +OX 2(g) and it is equal to ∆H=560\ \pu kJ ∆H = 560 kJ a) This reaction is: Endothermic or exothermic. b) Suppose 94.2\ \pu g 94.2 g of \ce Fe2O3 FeX 2OX 3 reacts. Will any heat be released or absorbed?

Source Image: tes.com
Download Image
Chemical Reaction Experiments You Can Easily Do at Home A chemist measures the energy change ΔH during the following reaction: 2NO2 (g)→N2O4 (g)=ΔH−55.3kJ Use the information to answer the following questions. Click the card to flip 👆 This reaction is..exothermic .Suppose 88.8g of NO2 react. Will any heat be released or absorbed? Yes, released.

Source Image: wardsworld.wardsci.com
Download Image
A Chemist Measures The Energy Change During The Following Reaction
A chemist measures the energy change ΔH during the following reaction: 2NO2 (g)→N2O4 (g)=ΔH−55.3kJ Use the information to answer the following questions. Click the card to flip 👆 This reaction is..exothermic .Suppose 88.8g of NO2 react. Will any heat be released or absorbed? Yes, released. Jul 18, 2023In an elementary laboratory, enthalpy changes are often measured in a “coffee-cup calorimeter” such as that shown in Figure 15.7.1 15.7. 1. Suppose the reaction to be measured is between two solutions. One of these solutions is introduced into the coffee cup, and the temperature of both solutions is measured.
Free endothermic freezer flask activity download
A chemist measures the energy change Δ H during the following reacti C H 4 ( g ) + 2 O 2 ( g ) → C O 2 ( g ) + 2 H 2 O ( I ) , Δ H = – 8 8 2 . k J Use the information to answer the following questions. From Code to Chemistry: Coscientist, the AI System Mastering Nobel Prize-Winning Reactions
Source Image: scitechdaily.com
Download Image
Lesson 6.7: Energy Changes in Chemical Reactions – American Chemical Society A chemist measures the energy change Δ H during the following reacti C H 4 ( g ) + 2 O 2 ( g ) → C O 2 ( g ) + 2 H 2 O ( I ) , Δ H = – 8 8 2 . k J Use the information to answer the following questions.
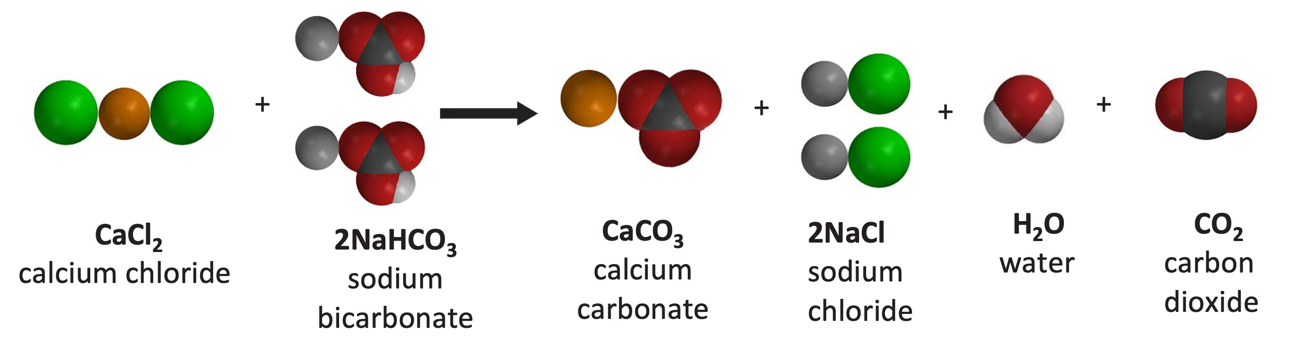
Source Image: acs.org
Download Image
QUIZ RATE OF REACTION Quiz Grade 12 Chemistry Quiz WITH ANSWERS Ver. #8 | Teaching Resources Assuming constant pressure, ΔH is a measure of the change of heat energy during a reaction. Since ΔH is negative, energy was released during the reaction therefore this reaction is exothermic. Suppose 20 g of H2O2 react. Will heat be released or absorbed?

Source Image: tes.com
Download Image
Chemical Reaction Experiments You Can Easily Do at Home A chemist measures the energy change ∆H during the following reaction \ce 2Fe2O3 (s)\rightarrow 4FeO (s)+O2 (g) 2FeX 2OX 3(s) → 4FeO(s)X +OX 2(g) and it is equal to ∆H=560\ \pu kJ ∆H = 560 kJ a) This reaction is: Endothermic or exothermic. b) Suppose 94.2\ \pu g 94.2 g of \ce Fe2O3 FeX 2OX 3 reacts. Will any heat be released or absorbed?

Source Image: educationpossible.com
Download Image
IB Chemistry IA Ideas and Research Questions Apr 12, 2023Energy is the capacity to do work. Mechanical work is the amount of energy required to move an object a given distance when opposed by a force. Thermal energy is due to the random motions of atoms, molecules, or ions in a substance. The temperature of an object is a measure of the amount of thermal energy it contains.

Source Image: chemistrytutor.me
Download Image
Premium Photo | Electrochemical cell or galvanic cell. the daniell cell is a primary voltaic cell with a copper anode and a zinc cathode. chemical energy change into electrical energy. A chemist measures the energy change ΔH during the following reaction: 2NO2 (g)→N2O4 (g)=ΔH−55.3kJ Use the information to answer the following questions. Click the card to flip 👆 This reaction is..exothermic .Suppose 88.8g of NO2 react. Will any heat be released or absorbed? Yes, released.

Source Image: freepik.com
Download Image
4+ Thousand Chemical Indicator Colour Royalty-Free Images, Stock Photos & Pictures | Shutterstock Jul 18, 2023In an elementary laboratory, enthalpy changes are often measured in a “coffee-cup calorimeter” such as that shown in Figure 15.7.1 15.7. 1. Suppose the reaction to be measured is between two solutions. One of these solutions is introduced into the coffee cup, and the temperature of both solutions is measured.

Source Image: shutterstock.com
Download Image
Lesson 6.7: Energy Changes in Chemical Reactions – American Chemical Society
4+ Thousand Chemical Indicator Colour Royalty-Free Images, Stock Photos & Pictures | Shutterstock This reaction released 48.7 kJ of heat when 3.12 g of glucose was burned. Exercise 5.3.5 5.3. 5. When 0.963 g of benzene, C 6 H 6, is burned in a bomb calorimeter, the temperature of the calorimeter increases by 8.39 °C. The bomb has a heat capacity of 784 J/°C and is submerged in 925 mL of water.
Chemical Reaction Experiments You Can Easily Do at Home Premium Photo | Electrochemical cell or galvanic cell. the daniell cell is a primary voltaic cell with a copper anode and a zinc cathode. chemical energy change into electrical energy. Apr 12, 2023Energy is the capacity to do work. Mechanical work is the amount of energy required to move an object a given distance when opposed by a force. Thermal energy is due to the random motions of atoms, molecules, or ions in a substance. The temperature of an object is a measure of the amount of thermal energy it contains.