Sep 22, 20222 HgO (s) → O 2 (g) + 2 Hg (l) 2 KClO 3 (s) → 3 O 2 (g) + 2 KCl (s) The potential products in double-replacement reactions are simple to predict; the anions and cations simply exchange. Remember, however, that one of the products must precipitate, otherwise no chemical reaction has occurred. For the reaction between lead (II) nitrate and
Identify the product in the following reaction.\n \n \n \n \n \n \n \n \n \n
Which among the following reactions is MOST likely to occur? … S A N 1 (Choice B) S A N 2 B. S A N 2 (Choice C) E1 C. E1 (Choice D) E2 D. E2 What is the MAJOR product formed in the given reaction? Choose all answers that apply: Choose all answers that apply: (Choice A) A (Choice B) B. Stuck? Review related articles

Source Image: youtube.com
Download Image
Determine the produced when reacts with H 2 according to the following reaction: H 2 ( g) + O 2 ( g) →, H 2 O ( l) There are 4 steps to solve this one.
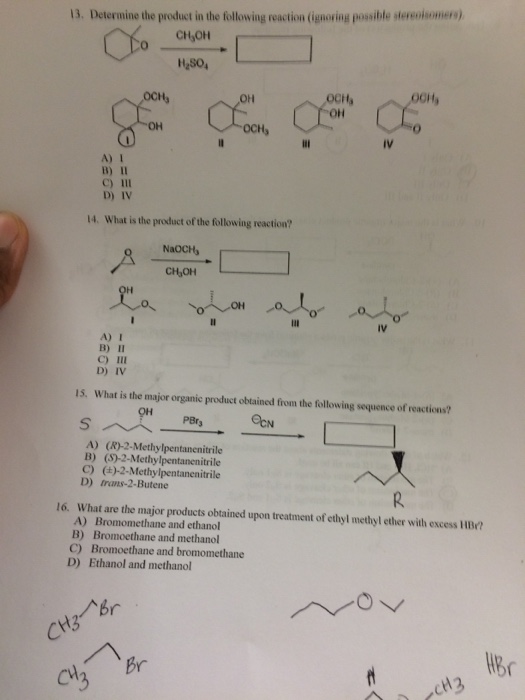
Source Image: chegg.com
Download Image
Solved 1) Predict the products of the following reactions. | Chegg.com A product is a substance that is present at the end of a chemical reaction. In the equation above, the zinc and sulfur are the reactants that chemically combine to form the zinc sulfide product. There is a standard way of writing chemical equations. The reactants are all written on the left-hand side of the equation, with the products on the
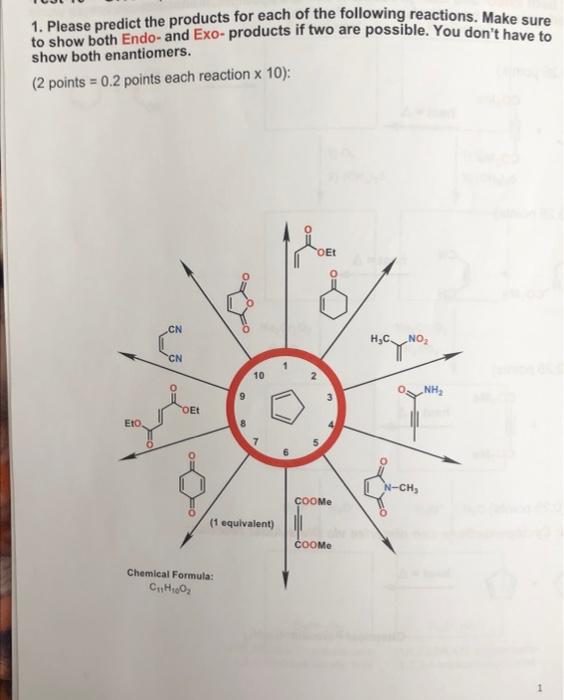
Source Image: chegg.com
Download Image
Determine The Products Of The Following Reaction
A product is a substance that is present at the end of a chemical reaction. In the equation above, the zinc and sulfur are the reactants that chemically combine to form the zinc sulfide product. There is a standard way of writing chemical equations. The reactants are all written on the left-hand side of the equation, with the products on the Not all double replacement reactions will occur ! In order for a double replacement reaction to take place: !Both of the reactants must be soluble in water !If a compound contains at least one of the ions that is proven soluble, then the compound will be at least moderately soluble !One product must be soluble and one product must be insoluble
Solved 1. Please predict the products for each of the | Chegg.com
Multiplying the number of moles of H A 2 SO A 4 by this factor gives us the number of moles of NaOH needed: 3.16 × 10 − 2 mol H 2 SO 4 × 2 mol NaOH 1 mol H 2 SO 4 = 6.32 × 10 − 2 mol NaOH. Notice how we wrote the mole ratio so that the moles of H A 2 SO A 4 cancel out, resulting in moles of NaOH as the final units. Identify the product Z in the following sequence of reactions phenol overset(NaOH)rarrX underset… – YouTube

Source Image: youtube.com
Download Image
Use Try On | Pinterest help Multiplying the number of moles of H A 2 SO A 4 by this factor gives us the number of moles of NaOH needed: 3.16 × 10 − 2 mol H 2 SO 4 × 2 mol NaOH 1 mol H 2 SO 4 = 6.32 × 10 − 2 mol NaOH. Notice how we wrote the mole ratio so that the moles of H A 2 SO A 4 cancel out, resulting in moles of NaOH as the final units.
Source Image: help.pinterest.com
Download Image
Identify the product in the following reaction.\n \n \n \n \n \n \n \n \n \n Sep 22, 20222 HgO (s) → O 2 (g) + 2 Hg (l) 2 KClO 3 (s) → 3 O 2 (g) + 2 KCl (s) The potential products in double-replacement reactions are simple to predict; the anions and cations simply exchange. Remember, however, that one of the products must precipitate, otherwise no chemical reaction has occurred. For the reaction between lead (II) nitrate and
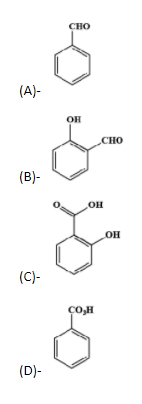
Source Image: vedantu.com
Download Image
Solved 1) Predict the products of the following reactions. | Chegg.com Determine the produced when reacts with H 2 according to the following reaction: H 2 ( g) + O 2 ( g) →, H 2 O ( l) There are 4 steps to solve this one.
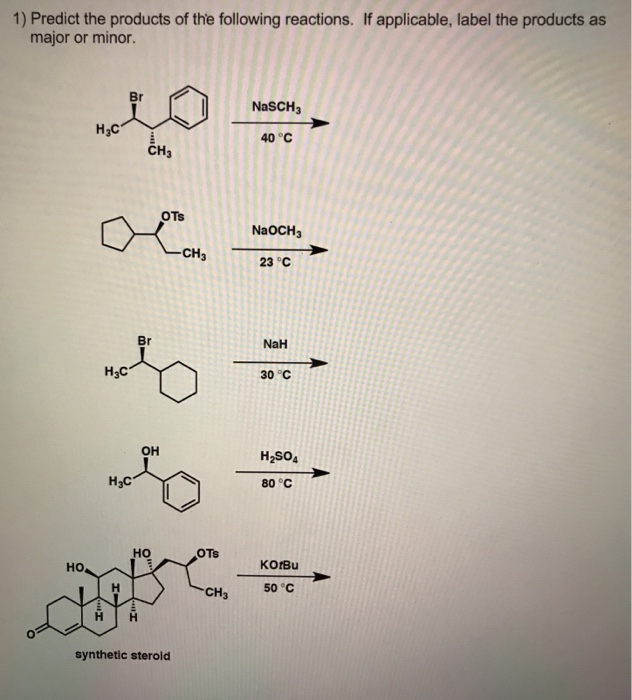
Source Image: chegg.com
Download Image
Solved 1. Draw the product in the following reactions; | Chegg.com Chemical Reaction Calculator. Calculator designed to balance chemical equations with results of: the balanced equation, word equation, and how it happened. Get the free “Chemical Reaction Calculator” widget for your website, blog, WordPress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
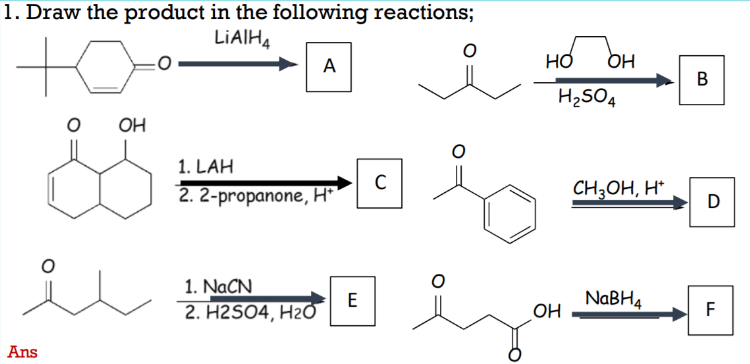
Source Image: chegg.com
Download Image
19.16: Additional Exercises – Chemistry LibreTexts A product is a substance that is present at the end of a chemical reaction. In the equation above, the zinc and sulfur are the reactants that chemically combine to form the zinc sulfide product. There is a standard way of writing chemical equations. The reactants are all written on the left-hand side of the equation, with the products on the
Source Image: chem.libretexts.org
Download Image
Solved Determine the products for each step of the following | Chegg.com Not all double replacement reactions will occur ! In order for a double replacement reaction to take place: !Both of the reactants must be soluble in water !If a compound contains at least one of the ions that is proven soluble, then the compound will be at least moderately soluble !One product must be soluble and one product must be insoluble
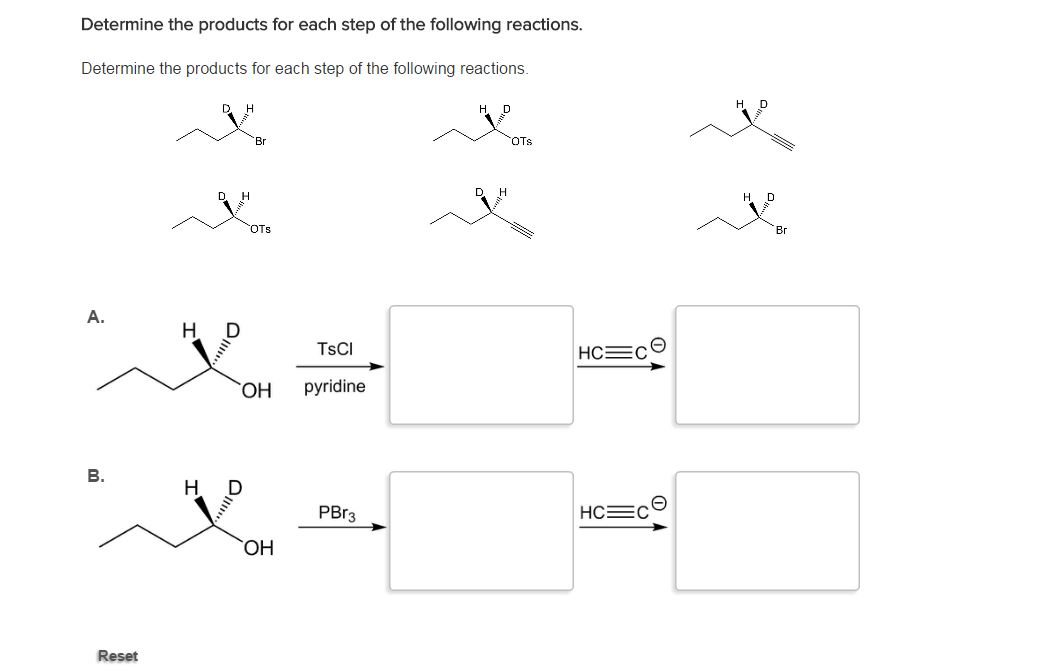
Source Image: chegg.com
Download Image
Use Try On | Pinterest help
Solved Determine the products for each step of the following | Chegg.com Which among the following reactions is MOST likely to occur? … S A N 1 (Choice B) S A N 2 B. S A N 2 (Choice C) E1 C. E1 (Choice D) E2 D. E2 What is the MAJOR product formed in the given reaction? Choose all answers that apply: Choose all answers that apply: (Choice A) A (Choice B) B. Stuck? Review related articles
Solved 1) Predict the products of the following reactions. | Chegg.com 19.16: Additional Exercises – Chemistry LibreTexts Chemical Reaction Calculator. Calculator designed to balance chemical equations with results of: the balanced equation, word equation, and how it happened. Get the free “Chemical Reaction Calculator” widget for your website, blog, WordPress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.