When you burn wood, the wood mixes with heat and oxygen to transform into carbon dioxide, water vapour, and ash. Once this change occurs, it cannot be returned to its original state. Chopping wood, on the other hand, is a physical change.
Chemical Reactions: Conditions & Speeds | Let’s Talk Science
When hydrogen bonds with oxygen, it produces water vapor — even as the wood burns. Fires burn only when all that atomic shuffling releases enough energy to keep the oxidation going in a sustained chain reaction. More atoms released from the fuel combine with nearby oxygen. That releases more energy, which releases more atoms.
Source Image: quora.com
Download Image
May 19, 2022CH4 + 2O2 —-> CO2 + 2H2O. The wood burns with oxygen to give carbon dioxide and water leaving ash as a residue. Since the atoms are balanced, the burning of wood has satisfied this requirement for being a chemical reaction.
Source Image: lambdageeks.com
Download Image
Decision to burn chemicals after Ohio derailment under scrutiny at hearing Flexi Says: Burning wood is a chemical change. When wood burns, it combines with oxygen and changes not only to ashes but also to carbon dioxide and water vapor. The gases float off into the air, leaving behind just the ashes. Discuss further with Flexi Ask your own question!

Source Image: youtube.com
Download Image
Why Is Burning Wood A Chemical Change
Flexi Says: Burning wood is a chemical change. When wood burns, it combines with oxygen and changes not only to ashes but also to carbon dioxide and water vapor. The gases float off into the air, leaving behind just the ashes. Discuss further with Flexi Ask your own question! Oct 19, 2023Matter is capable of undergoing changes, which are classified as either physical or chemical. Physical changes in matter are often reversible: An ice cube can melt into liquid water, and then the liquid water can be frozen back into an ice cube. Chemical changes, on the other hand, are not reversible: A log burned in a fire turns to ashes, but
Is Burning Wood a Chemical or Physical Change? – YouTube
Burning wood is a chemical reaction that occurs when wood is exposed to heat and oxygen. This process, known as combustion, releases energy in the form of heat and light. Wood is primarily composed of cellulose, hemicellulose, and lignin, which are organic compounds. Physical and Chemical Changes – Water Boiling and Water Electrolysis. Stock Vector – Illustration of electrolysis, children: 94216160
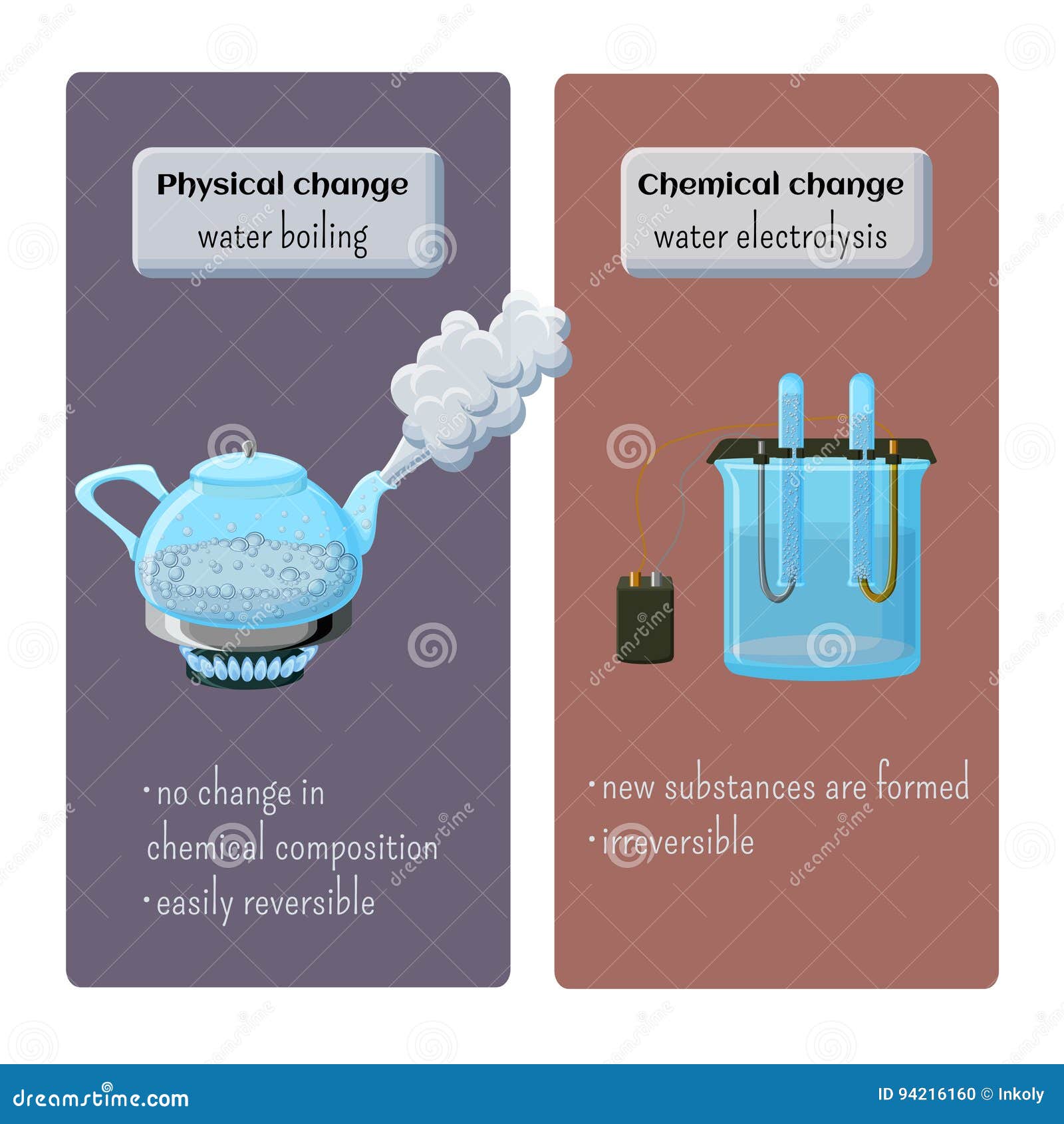
Source Image: dreamstime.com
Download Image
Chemical Reaction Smoke Stock Illustrations – 633 Chemical Reaction Smoke Stock Illustrations, Vectors & Clipart – Dreamstime Burning wood is a chemical reaction that occurs when wood is exposed to heat and oxygen. This process, known as combustion, releases energy in the form of heat and light. Wood is primarily composed of cellulose, hemicellulose, and lignin, which are organic compounds.
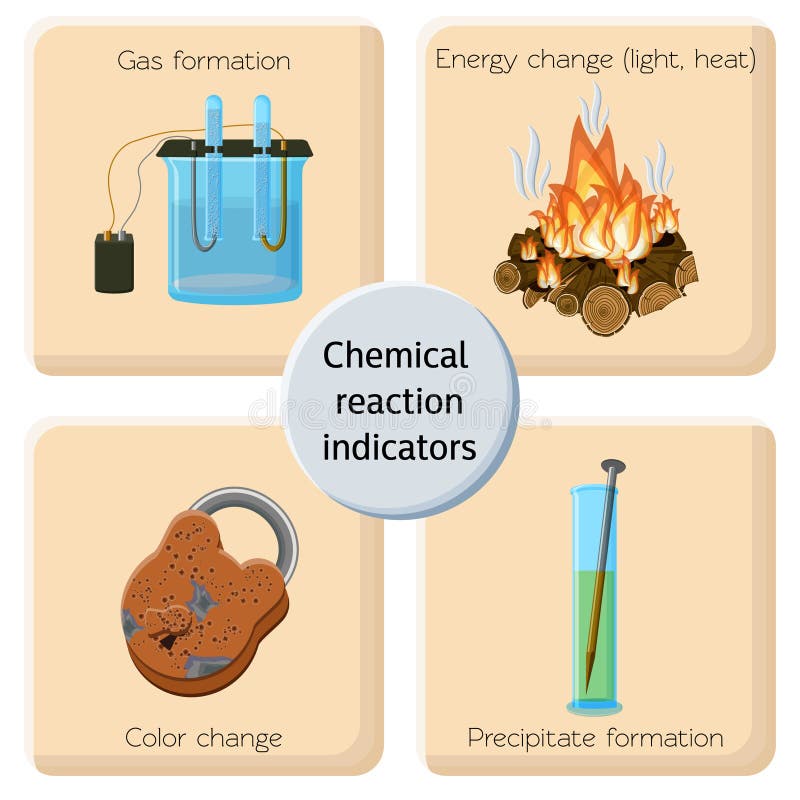
Source Image: dreamstime.com
Download Image
Chemical Reactions: Conditions & Speeds | Let’s Talk Science When you burn wood, the wood mixes with heat and oxygen to transform into carbon dioxide, water vapour, and ash. Once this change occurs, it cannot be returned to its original state. Chopping wood, on the other hand, is a physical change.
Source Image: letstalkscience.ca
Download Image
Decision to burn chemicals after Ohio derailment under scrutiny at hearing May 19, 2022CH4 + 2O2 —-> CO2 + 2H2O. The wood burns with oxygen to give carbon dioxide and water leaving ash as a residue. Since the atoms are balanced, the burning of wood has satisfied this requirement for being a chemical reaction.

Source Image: nbcnews.com
Download Image
How to Use Your Log Burner Vents – Direct Stoves | Direct Stoves Aug 28, 2022August 28, 2022 by Jace Mark When you burn wood, it undergoes a chemical change. The heat from the fire causes the molecules in the wood to break down, releasing energy in the form of light and heat. This process is called combustion. Wood burning is often thought of as a physical change, but it is actually a chemical change.

Source Image: directstoves.com
Download Image
Evidence of Chemical Change | Let’s Talk Science Flexi Says: Burning wood is a chemical change. When wood burns, it combines with oxygen and changes not only to ashes but also to carbon dioxide and water vapor. The gases float off into the air, leaving behind just the ashes. Discuss further with Flexi Ask your own question!

Source Image: letstalkscience.ca
Download Image
Examples of Chemical Change – Carolina Knowledge Center Oct 19, 2023Matter is capable of undergoing changes, which are classified as either physical or chemical. Physical changes in matter are often reversible: An ice cube can melt into liquid water, and then the liquid water can be frozen back into an ice cube. Chemical changes, on the other hand, are not reversible: A log burned in a fire turns to ashes, but

Source Image: knowledge.carolina.com
Download Image
Chemical Reaction Smoke Stock Illustrations – 633 Chemical Reaction Smoke Stock Illustrations, Vectors & Clipart – Dreamstime
Examples of Chemical Change – Carolina Knowledge Center When hydrogen bonds with oxygen, it produces water vapor — even as the wood burns. Fires burn only when all that atomic shuffling releases enough energy to keep the oxidation going in a sustained chain reaction. More atoms released from the fuel combine with nearby oxygen. That releases more energy, which releases more atoms.
Decision to burn chemicals after Ohio derailment under scrutiny at hearing Evidence of Chemical Change | Let’s Talk Science Aug 28, 2022August 28, 2022 by Jace Mark When you burn wood, it undergoes a chemical change. The heat from the fire causes the molecules in the wood to break down, releasing energy in the form of light and heat. This process is called combustion. Wood burning is often thought of as a physical change, but it is actually a chemical change.