The formula for the ionic compound made entirely of aluminum and oxygen can be determined by considering the charges of the ions involved. Aluminum typically forms a 3+ cation (Al3+), while oxygen forms a 2- anion (O2-). To balance the charges, we need to have three oxygen ions for every aluminum ion.
Type of Reaction for Al + O2 = Al2O3 – YouTube
Part A Aluminum metal reacts with oxygen gas to form aluminum oxide. Express your answer as a chemical equation. ΑΣΦ ? ха a b хь a b . – х ex 11 A chemical reaction does not occur for this question. Submit Previous Answers Request Answer Part B Calcium carbonate (the active ingredient in many antacids) Show transcribed image text
Source Image: freepik.com
Download Image
Jun 12, 2023The chemical formula for a compound of aluminum and oxygen is Al2O3, while the formula for a compound of beryllium and iodine is BeI2. Explanation: When aluminum interacts with oxygen, the resulting compound is Aluminum oxide (Al2O3). This is due to the fact that aluminum has an oxidation state of 3+, and oxygen has an oxidation state of 2-.

Source Image: shutterstock.com
Download Image
How to Balance: Al + O2 = Al2O3 – YouTube Jul 3, 2014 The formula for aluminum oxide is Al2O3. Explanation: The correct answer is Al2O3. Let us see how we got the answer; Look at the electronic arrangement of Al and O atoms. Al ( Z= 13) has 13 electrons with following electronic configuration. 1 s2 2 s2 2 p6 3 s2 3 p1

Source Image: youtube.com
Download Image
Aluminum And Oxygen Express Your Answer As A Chemical Formula
Jul 3, 2014 The formula for aluminum oxide is Al2O3. Explanation: The correct answer is Al2O3. Let us see how we got the answer; Look at the electronic arrangement of Al and O atoms. Al ( Z= 13) has 13 electrons with following electronic configuration. 1 s2 2 s2 2 p6 3 s2 3 p1 Aluminium Oxide Chemical Formula. The formula of aluminium oxide is given as Al 2 O 3. How is this formula generated? First of all, we have to consider that aluminium is a metal and Oxygen is a non-metal making it an ionic compound. There is some charge in the aluminium and oxygen ions.
Balance the chemical equation. Al + O2 = Al2O3. Aluminum+oxygen=aluminium oxide. – YouTube
Step 4: Substitute Coefficients and Verify Result. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. 2 Al2O3 = 4 Al + 3 O2. Reactants. Products. PPT – (i) Name the process involved in this experiment PowerPoint Presentation – ID:5979342

Source Image: slideserve.com
Download Image
Formula for Aluminum oxide – YouTube Step 4: Substitute Coefficients and Verify Result. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. 2 Al2O3 = 4 Al + 3 O2. Reactants. Products.

Source Image: youtube.com
Download Image
Type of Reaction for Al + O2 = Al2O3 – YouTube The formula for the ionic compound made entirely of aluminum and oxygen can be determined by considering the charges of the ions involved. Aluminum typically forms a 3+ cation (Al3+), while oxygen forms a 2- anion (O2-). To balance the charges, we need to have three oxygen ions for every aluminum ion.

Source Image: youtube.com
Download Image
How to Balance: Al + O2 = Al2O3 – YouTube Jun 12, 2023The chemical formula for a compound of aluminum and oxygen is Al2O3, while the formula for a compound of beryllium and iodine is BeI2. Explanation: When aluminum interacts with oxygen, the resulting compound is Aluminum oxide (Al2O3). This is due to the fact that aluminum has an oxidation state of 3+, and oxygen has an oxidation state of 2-.

Source Image: youtube.com
Download Image
Balancing the Equation Al + O2 = Al2O3 (and Type of Reaction) – YouTube Chemistry Chemistry questions and answers Part A aluminum and oxygen Express your answer as a chemical formula. | ΑΣΦ ? Submit Request Answer Part B beryllium and iodine Express your answer as a chemical formula. ΑΣΦ Submit Request Answer Part 4 Ο «εν This problem has been solved!

Source Image: m.youtube.com
Download Image
Oxygen symbol chemical element of the periodic Vector Image Jul 3, 2014 The formula for aluminum oxide is Al2O3. Explanation: The correct answer is Al2O3. Let us see how we got the answer; Look at the electronic arrangement of Al and O atoms. Al ( Z= 13) has 13 electrons with following electronic configuration. 1 s2 2 s2 2 p6 3 s2 3 p1

Source Image: vectorstock.com
Download Image
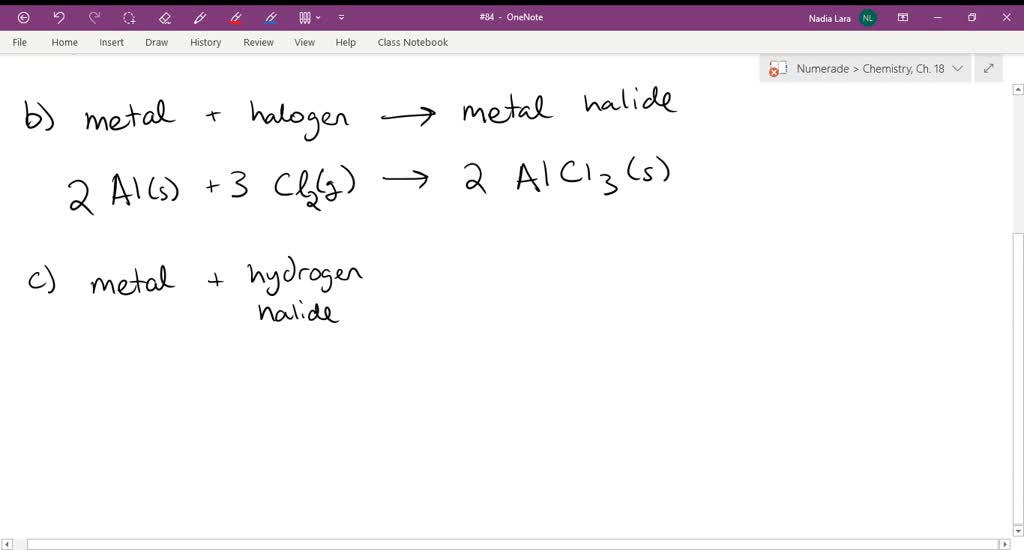
⏩SOLVED:Write balanced chemical equations for the following… | Numerade Aluminium Oxide Chemical Formula. The formula of aluminium oxide is given as Al 2 O 3. How is this formula generated? First of all, we have to consider that aluminium is a metal and Oxygen is a non-metal making it an ionic compound. There is some charge in the aluminium and oxygen ions.
Source Image: numerade.com
Download Image
Formula for Aluminum oxide – YouTube
⏩SOLVED:Write balanced chemical equations for the following… | Numerade Part A Aluminum metal reacts with oxygen gas to form aluminum oxide. Express your answer as a chemical equation. ΑΣΦ ? ха a b хь a b . – х ex 11 A chemical reaction does not occur for this question. Submit Previous Answers Request Answer Part B Calcium carbonate (the active ingredient in many antacids) Show transcribed image text
How to Balance: Al + O2 = Al2O3 – YouTube Oxygen symbol chemical element of the periodic Vector Image Chemistry Chemistry questions and answers Part A aluminum and oxygen Express your answer as a chemical formula. | ΑΣΦ ? Submit Request Answer Part B beryllium and iodine Express your answer as a chemical formula. ΑΣΦ Submit Request Answer Part 4 Ο «εν This problem has been solved!